What is the relationship between frequency and energy
The relationship between frequency and energy is fundamental in physics, particularly in the study of electromagnetic waves and quantum mechanics. Energy and frequency are directly proportional to each other, as described by Planck’s equation:
E=hfE = h
where:
- EE is the energy of the wave or photon,
- hh is Planck’s constant (6.626×10−34 Js6.626 \times 10^{-34} \, \text{Js}),
- ff (or ν\nu) is the frequency of the wave.
This equation shows that as the frequency of a wave increases, its energy also increases. This principle applies to all electromagnetic waves, including radio waves, microwaves, infrared, visible light, ultraviolet, X-rays, and gamma rays.
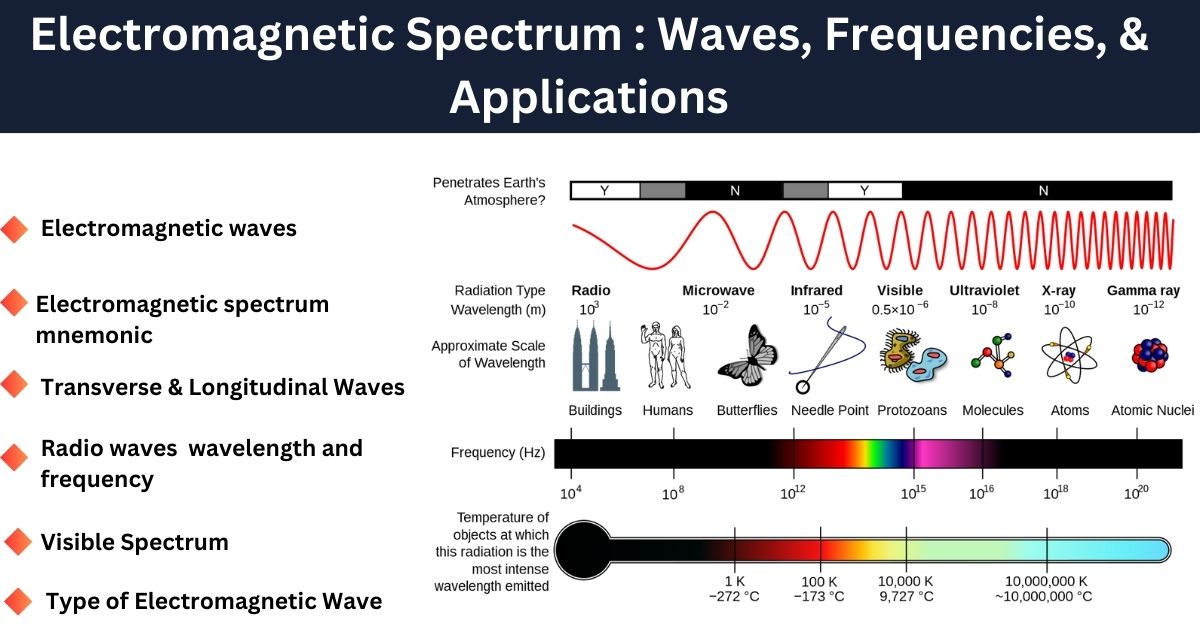
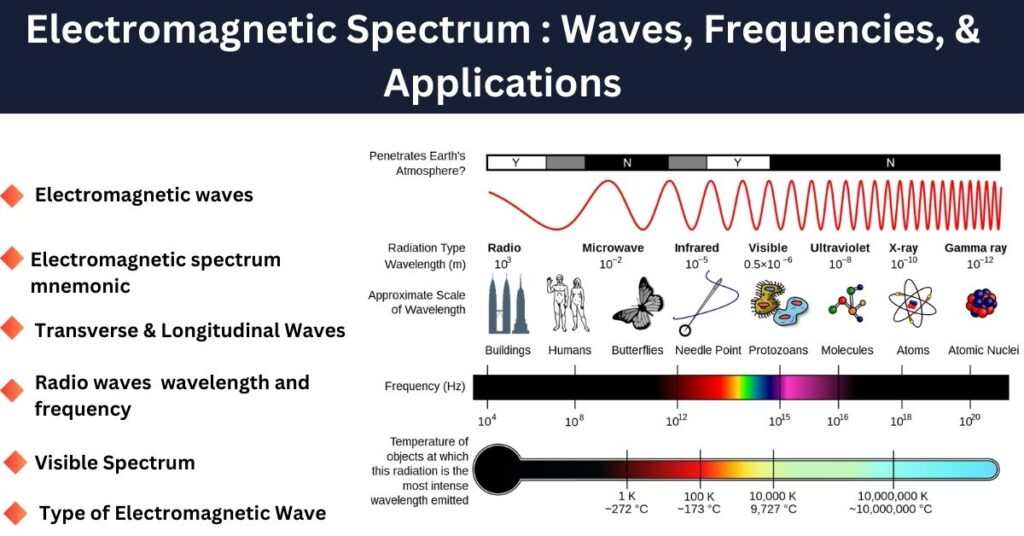
In the electromagnetic spectrum, low-frequency waves like radio waves have lower energy, while high-frequency waves like X-rays and gamma rays have higher energy. For example, visible light has a frequency range of approximately 4×10144 \times 10^{14} Hz (red) to 7.5×10147.5 \times 10^{14} Hz (violet).
Ultraviolet (UV) light has a higher frequency than visible light, which gives it more energy, making it capable of causing chemical reactions and even harming biological tissues.
This relationship is crucial in quantum mechanics, where light and electromagnetic radiation exhibit both particle and wave properties. The quantization of energy levels in atoms is explained by this frequency-energy relationship.
When an electron moves from a higher to a lower energy level, it emits a photon whose energy is determined by the frequency of the emitted radiation. Conversely, absorbing a photon of the correct frequency can excite an electron to a higher energy state.
In practical applications, this concept is used in technologies such as lasers, medical imaging (X-rays), and radiation therapy.
Higher-frequency waves have higher energy and can penetrate materials more effectively, which is why gamma rays are used for sterilization and cancer treatment. Similarly, in communication systems, lower-frequency radio waves are used for long-distance transmission because they carry less energy and suffer less attenuation.
The relationship between frequency and energy is also evident in thermal radiation. According to Planck’s law, the energy radiated by a black body depends on the frequency of the emitted waves.
Hotter objects emit higher-frequency radiation, which is why the color of heated metal changes from red to white as the temperature increases. The Wien’s displacement law further states that the peak emission wavelength shifts toward shorter wavelengths as the temperature rises.
Understanding this frequency-energy relationship is essential in multiple scientific fields, including astrophysics, where it helps in studying cosmic radiation and stellar emissions.
The energy of photons from celestial bodies provides insights into their temperature, composition, and movement. High-energy gamma-ray bursts, for instance, are among the most energetic phenomena observed in space.
In nuclear physics, the energy released in radioactive decay is also linked to frequency. Gamma decay involves the emission of high-frequency photons, releasing energy from an excited atomic nucleus. This principle is utilized in nuclear medicine and industrial radiography for material testing.
Key Points:
- Direct Proportionality – Energy and frequency are directly proportional, as given by Planck’s equation: E=hfE = h f. When frequency increases, energy also increases.
- Electromagnetic Spectrum – Lower-frequency waves like radio waves have lower energy, while higher-frequency waves like X-rays and gamma rays have higher energy.
- Quantum Mechanics – In atoms, electron transitions involve absorption or emission of photons, where the energy of a photon depends on its frequency.
- Medical Applications – High-frequency waves like X-rays and gamma rays are used in medical imaging and cancer treatment due to their high energy.
- Thermal Radiation – Hotter objects emit higher-frequency radiation, as described by Planck’s law and Wien’s displacement law.
- Astrophysics – High-energy cosmic radiation and gamma-ray bursts are studied based on the frequency-energy relationship.
- Nuclear Physics – In radioactive decay, gamma rays with high frequency and energy are emitted during nuclear transitions.
- Communication Systems – Low-frequency radio waves are used for long-distance communication because they carry less energy and experience less attenuation.
- Material Interaction – High-frequency waves penetrate materials more effectively, which is why X-rays are used for imaging bones and security scanning.
- Lasers and Photonics – Laser technology relies on precise energy-frequency relationships to produce coherent light for medical, industrial, and communication applications.
how fast do electromagnetic waves travel
Electromagnetic waves travel at the speed of light, which is approximately 299,792,458 meters per second (m/s) in a vacuum. This speed is often rounded to 3.0×1083.0 \times 10^8 m/s for simplicity.
The speed of electromagnetic waves depends on the medium they travel through. In air, they move at nearly the same speed as in a vacuum. However, in denser materials like water, glass, or optical fibers, their speed decreases due to interactions with the medium’s atoms. The reduction in speed is determined by the refractive index (nn) of the material, given by the formula: v=cnv = \frac{c}{n}
where:
- vv is the speed of the wave in the medium,
- cc is the speed of light in a vacuum,
- nn is the refractive index of the material.
For example, in water (n≈1.33n \approx 1.33), the speed of light reduces to around 2.25×1082.25 \times 10^8 m/s. In glass (n≈1.5n \approx 1.5), it slows down further to about 2.0×1082.0 \times 10^8 m/s.
Despite these variations, electromagnetic waves always move faster than sound waves, which travel at about 343 m/s in air. This vast difference explains why we see lightning before hearing thunder.
Key Points:
- Speed in Vacuum – Electromagnetic waves travel at 3.0×1083.0 \times 10^8 m/s in a vacuum, the fastest possible speed in the universe.
- Medium Dependence – Their speed decreases in materials like water, glass, and fiber optics due to the refractive index.
- Formula for Speed in Medium – The speed of electromagnetic waves in a medium is given by v=cnv = \frac{c}{n}, where nn is the refractive index.
- Speed in Air – Nearly equal to their speed in a vacuum because air’s refractive index is close to 1.
- Speed in Water – Around 2.25×1082.25 \times 10^8 m/s, as water’s refractive index is 1.33.
- Speed in Glass – Reduced further to about 2.0×1082.0 \times 10^8 m/s, depending on the glass type.
- Comparison with Sound Waves – Electromagnetic waves are much faster than sound, which travels at 343 m/s in air.
- Practical Applications – This property is crucial in fiber optic communication, satellite transmissions, and wireless technologies.
- Astronomical Significance – Light from the Sun takes about 8 minutes and 20 seconds to reach Earth, demonstrating the immense speed of electromagnetic waves.
- Lightning and Thunder – Since light travels faster than sound, we see lightning before hearing thunder.
electromagnetic spectrum mnemonic
A popular mnemonic to remember the order of the electromagnetic spectrum from low frequency (long wavelength, low energy) to high frequency (short wavelength, high energy) is:

“Rabbits Mate In Very Unusual eXpensive Gardens.”
Where:
- R – Radio Waves (Lowest Frequency, Longest Wavelength)
- M – Microwaves
- I – Infrared
- V – Visible Light
- U – Ultraviolet
- X – X-rays
- G – Gamma Rays (Highest Frequency, Shortest Wavelength)
If you prefer a variation, another mnemonic is:
“Rich Men In Vegas Use X-ray Glasses.”
Both help in memorizing the sequence easily! 🚀
is the electromagnetic spectrum transverse or longitudinal
The electromagnetic spectrum consists of transverse waves. In electromagnetic waves, the electric field and magnetic field oscillate perpendicular to each other and to the direction of wave propagation. This means that the wave moves forward while the fields vibrate at right angles to the direction of motion.
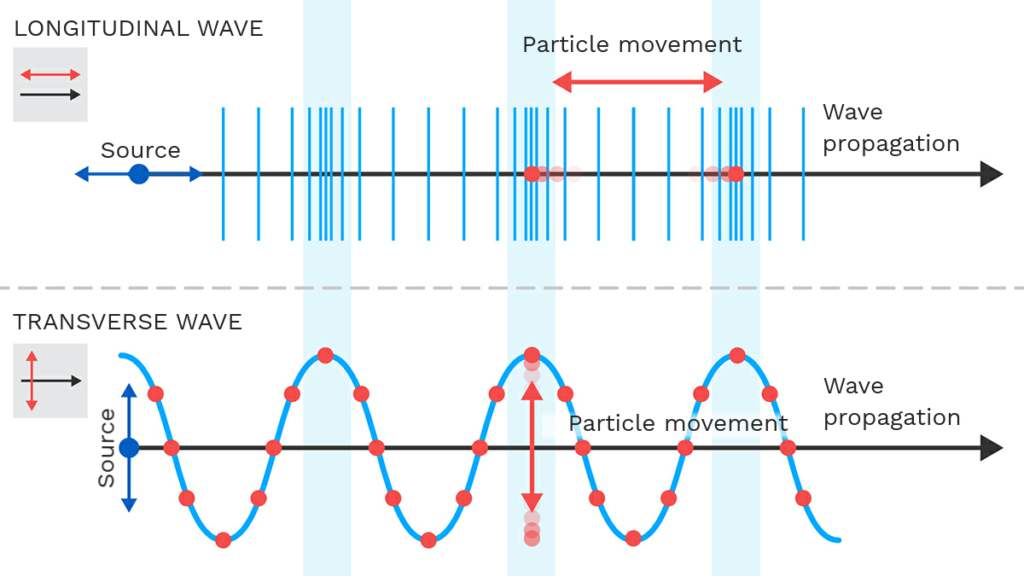
Unlike longitudinal waves (such as sound waves), electromagnetic waves do not require a medium and can travel through the vacuum of space. This is why light from the Sun and distant stars reaches Earth.
which part of the electromagnetic spectrum has the longest wavelengths
Radio waves occupy the longest wavelengths in the electromagnetic spectrum, ranging from about 1 millimeter to thousands of kilometers. These waves have the lowest frequency and least energy among all electromagnetic waves.
Why Do Radio Waves Have the Longest Wavelengths?
The wavelength of an electromagnetic wave is inversely proportional to its frequency, as given by the wave equation: c=λfc = \lambda f
where:
- cc is the speed of light (3.0×1083.0 \times 10^8 m/s),
- λ\lambda (lambda) is the wavelength in meters,
- ff is the frequency in hertz (Hz).
Since radio waves have the lowest frequency, they must have the longest wavelength to maintain this relationship.
Types of Radio Waves Based on Wavelength
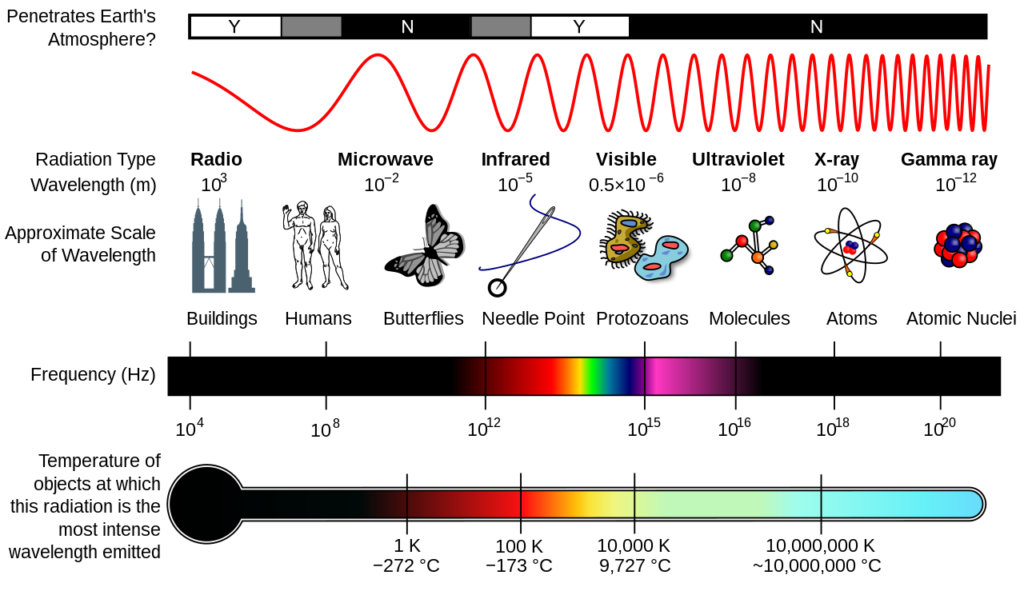
Radio waves are further classified based on their wavelength and frequency:
- Extremely Low Frequency (ELF) – Wavelength: >1000 km, used for submarine communication.
- Very Low Frequency (VLF) – Wavelength: 10 km – 1000 km, used in navigation and radio communication.
- Low Frequency (LF) – Wavelength: 1 km – 10 km, used for long-range radio navigation.
- Medium Frequency (MF) – Wavelength: 100 m – 1 km, used in AM radio broadcasting.
- High Frequency (HF) – Wavelength: 10 m – 100 m, used in shortwave radio and aviation communication.
- Very High Frequency (VHF) – Wavelength: 1 m – 10 m, used in FM radio and TV broadcasts.
- Ultra High Frequency (UHF) – Wavelength: 10 cm – 1 m, used in mobile phones and GPS.
- Microwave Range – Wavelength: 1 mm – 10 cm, used in radar, satellite communication, and cooking.
Video Source : AnandSystemsEngineering ( Youtube)
Characteristics of Long-Wavelength Radio Waves
- Low Energy: Due to their low frequency, radio waves carry very little energy compared to other electromagnetic waves.
- Long-Distance Travel: They can travel vast distances with minimal energy loss, making them ideal for communication.
- Penetration Through Obstacles: Long-wavelength radio waves can diffract around obstacles like buildings and mountains.
- Ionospheric Reflection: Some radio waves (like AM waves) reflect off the Earth’s ionosphere, enabling long-distance transmission.
Applications of Long-Wavelength Radio Waves
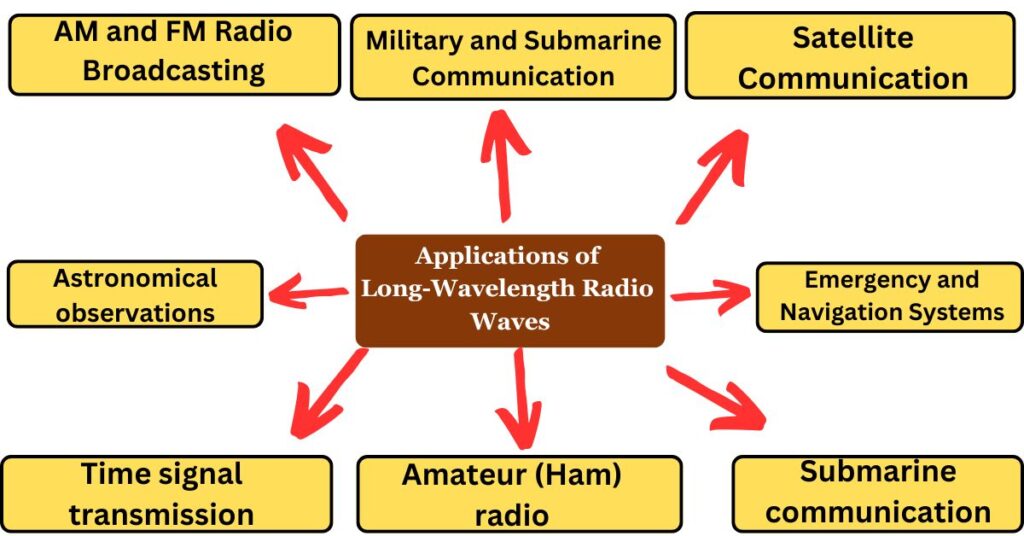
- AM and FM Radio Broadcasting – AM radio uses medium frequencies, while FM radio uses very high frequencies.
- Military and Submarine Communication – ELF and VLF waves penetrate deep into water, making them useful for underwater communication.
- Satellite Communication – Certain radio waves are used in deep-space communication, allowing contact with distant spacecraft.
- Emergency and Navigation Systems – Long-wave signals help in maritime and aviation navigation.
Comparison With Other Electromagnetic Waves
- Microwaves have shorter wavelengths than radio waves but are still relatively long.
- Infrared, visible light, ultraviolet, X-rays, and gamma rays have much shorter wavelengths and higher frequencies, carrying more energy.
Thus, radio waves are the longest electromagnetic waves, making them crucial for communication and navigation technologies.
are sound waves part of the electromagnetic spectrum
No, sound waves are not part of the electromagnetic spectrum. The electromagnetic spectrum consists of waves such as radio waves, microwaves, infrared, visible light, ultraviolet, X-rays, and gamma rays, which do not require a medium to travel and can propagate through a vacuum.
Key Differences Between Sound Waves and Electromagnetic Waves
- Nature of Waves
- Sound waves are mechanical waves, meaning they require a medium (air, water, or solids) to travel.
- Electromagnetic waves are transverse waves that can travel through a vacuum, as they consist of oscillating electric and magnetic fields.
- Type of Propagation
- Sound waves are longitudinal waves, where particles in the medium vibrate parallel to the wave’s direction.
- Electromagnetic waves are transverse waves, where electric and magnetic fields oscillate perpendicular to each other and to the direction of wave propagation.
- Speed of Travel
- Sound waves travel much slower, at about 343 m/s in air and vary depending on the medium (faster in solids, slower in gases).
- Electromagnetic waves travel at 3.0×1083.0 \times 10^8 m/s in a vacuum, which is nearly a million times faster than sound.
- Medium Dependency
- Sound waves need a medium (solid, liquid, or gas) and cannot travel in a vacuum.
- Electromagnetic waves do not require a medium and can propagate through space.
- Energy Transfer
- Sound waves transfer mechanical energy through compressions and rarefactions in the medium.
- Electromagnetic waves carry electromagnetic energy, which can travel without a medium.
- Frequency and Wavelength Range
- Sound waves typically have frequencies between 20 Hz to 20 kHz (audible range), with wavelengths ranging from centimeters to meters.
- Electromagnetic waves have an extremely wide range of frequencies and wavelengths, from long-wavelength radio waves to short-wavelength gamma rays.
Why Sound Waves Are Not in the Electromagnetic Spectrum
Since sound requires a medium and moves via particle vibrations, it does not belong to the electromagnetic spectrum, which consists only of waves generated by oscillating electric and magnetic fields. This fundamental difference separates mechanical waves (sound) from electromagnetic waves (light, radio, X-rays, etc.).
What portion of the electromagnetic spectrum is visible
The visible portion of the electromagnetic spectrum is the range of wavelengths that the human eye can detect, typically from 400 nm to 700 nm (nanometers). This range lies between infrared (IR) and ultraviolet (UV) radiation.
Characteristics of the Visible Spectrum
- Wavelength Range: Approximately 400 nm (violet) to 700 nm (red).
- Frequency Range: Around 4.3×10144.3 \times 10^{14} Hz to 7.5×10147.5 \times 10^{14} Hz.
- Energy Range: About 1.77 eV to 3.1 eV (electron volts).
Colors in the Visible Spectrum
The visible spectrum consists of different colors, each corresponding to a specific wavelength:
- Violet: ~400 nm – Shortest wavelength, highest frequency, and highest energy.
- Blue: ~450 nm.
- Green: ~500 nm.
- Yellow: ~570 nm.
- Orange: ~600 nm.
- Red: ~700 nm – Longest wavelength, lowest frequency, and lowest energy.
Why Is It Called the Visible Spectrum?
The human eye contains cone cells that are sensitive to this range of wavelengths, allowing us to perceive different colors. Beyond this range:
- Infrared (IR) waves (longer than 700 nm) are invisible but can be felt as heat.
- Ultraviolet (UV) waves (shorter than 400 nm) are also invisible and can cause sunburn.
Applications of the Visible Spectrum
- Human Vision – Enables us to see objects and colors.
- Optical Communication – Used in fiber optics and lasers.
- Photography and Imaging – Cameras detect visible light to capture images.
- Astronomy – Telescopes analyze starlight to study celestial objects.
Thus, the visible spectrum is a small but essential part of the vast electromagnetic spectrum, allowing humans to perceive the world in color.
No, higher energy photons have shorter wavelengths.
According to the equation for photon energy: E=hfE = hf
where:
- EE is the photon energy,
- hh is Planck’s constant (6.626×10−34 J\cdotps6.626 \times 10^{-34} \, \text{J·s}),
- ff is the frequency of the wave.
Since frequency and wavelength are related by the equation: c=λfc = \lambda f
where cc is the speed of light, we can see that: E=hcλE = \frac{hc}{\lambda}
From this equation, energy (EE) is inversely proportional to wavelength (λ\lambda). This means:
- Higher energy photons have shorter wavelengths (e.g., X-rays and gamma rays).
- Lower energy photons have longer wavelengths (e.g., radio waves and microwaves).
For example:
- Gamma rays (highest energy) have wavelengths less than 0.01 nm.
- Radio waves (lowest energy) have wavelengths longer than 1 meter.
Thus, as photon energy increases, wavelength decreases.
do all electromagnetic waves travel at the same speed
In a vacuum, all electromagnetic waves travel at the same speed, which is the speed of light (3.0×1083.0 \times 10^8 m/s).
However, when electromagnetic waves travel through a medium (such as air, water, or glass), their speed varies depending on the material’s refractive index.
Electromagnetic Wave Speed in Different Mediums
The speed of an electromagnetic wave in a medium is given by: v=cnv = \frac{c}{n}
where:
- vv = speed of light in the medium,
- cc = speed of light in a vacuum (3.0×1083.0 \times 10^8 m/s),
- nn = refractive index of the medium.
Effect of Medium on Wave Speed
- In a vacuum: All electromagnetic waves travel at 3.0×1083.0 \times 10^8 m/s.
- In air: Speed is slightly less than in a vacuum, but nearly the same.
- In water: Light slows down to about 2.25×1082.25 \times 10^8 m/s (n≈1.33n \approx 1.33).
- In glass: Light slows down further to around 2.0×1082.0 \times 10^8 m/s (n≈1.5n \approx 1.5).
- In dense materials (e.g., diamond): Light slows significantly to 1.25×1081.25 \times 10^8 m/s (n≈2.4n \approx 2.4).
Does Wavelength Affect Speed?
In a vacuum, wavelength does not affect speed, so all electromagnetic waves—radio waves, visible light, X-rays, gamma rays—travel at the same speed. However, in a medium, different wavelengths can slow down by different amounts due to dispersion (e.g., visible light splitting into colors in a prism).
how are the frequency and wavelength of light related
The frequency and wavelength of light are inversely related according to the wave equation: c=λfc = \lambda f
where:
- cc = speed of light in a vacuum (3.0×1083.0 \times 10^8 m/s),
- λ\lambda = wavelength (in meters),
- ff = frequency (in hertz, Hz).
Relationship Between Frequency and Wavelength
- If the frequency increases, the wavelength decreases.
- If the frequency decreases, the wavelength increases.
Example Calculations
For red light (λ≈700\lambda \approx 700 nm): f=cλ=3.0×108700×10−9f = \frac{c}{\lambda} = \frac{3.0 \times 10^8}{700 \times 10^{-9}} f≈4.3×1014 Hzf \approx 4.3 \times 10^{14} \text{ Hz}
For blue light (λ≈400\lambda \approx 400 nm): f=3.0×108400×10−9f = \frac{3.0 \times 10^8}{400 \times 10^{-9}} f≈7.5×1014 Hzf \approx 7.5 \times 10^{14} \text{ Hz}
Effect on Energy
Since photon energy is given by: E=hfE = hf
where hh is Planck’s constant (6.626×10−346.626 \times 10^{-34} J·s), higher frequency waves have more energy, while lower frequency waves have less energy.
This explains why ultraviolet (UV) light is more energetic than infrared (IR) light and why gamma rays are more harmful than radio waves.
Wavelength and Frequency of Light Colors
| Color | Wavelength (nm) | Frequency (THz) |
|---|---|---|
| Violet | 380 – 450 | 668 – 789 |
| Blue | 450 – 495 | 606 – 668 |
| Green | 495 – 570 | 526 – 606 |
| Yellow | 570 – 590 | 508 – 526 |
| Orange | 590 – 620 | 484 – 508 |
| Red | 620 – 750 | 400 – 484 |
what is the longest wavelength
Longest Wavelength
The longest wavelength in the electromagnetic spectrum belongs to radio waves. These waves can have wavelengths ranging from 1 millimeter to thousands of kilometers.
For example:
- AM radio waves: ~300 meters
- FM radio waves: ~3 meters
- Some deep-space signals: over 100 kilometers
what is the shortest wavelength
Shortest Wavelength
The shortest wavelength belongs to gamma rays. These waves have wavelengths smaller than 0.01 nanometers (nm), often in the picometer (pm) range.
For example:
- Gamma rays from nuclear reactions: ~0.01 nm
- High-energy cosmic gamma rays: less than 0.001 nm
Thus, radio waves have the longest wavelengths, while gamma rays have the shortest wavelengths.
what type of wave is a microwave
Microwaves – A Type of Electromagnetic Wave
Microwaves are electromagnetic waves with wavelengths ranging from 1 millimeter to 1 meter and frequencies between 300 MHz and 300 GHz. They fall between radio waves and infrared waves in the electromagnetic spectrum.
Characteristics of Microwaves
| Property | Description |
|---|---|
| Type of Wave | Electromagnetic, Transverse |
| Wavelength Range | 1 mm to 1 m |
| Frequency Range | 300 MHz to 300 GHz |
| Travel Medium | Can travel through a vacuum (does not require a medium) |
| Energy Level | Higher than radio waves, lower than infrared waves |
| Speed in Vacuum | 3.0×1083.0 \times 10^8 m/s (same as all electromagnetic waves) |
How Microwaves Work
Microwaves are transverse electromagnetic waves, meaning they consist of oscillating electric and magnetic fields perpendicular to each other and the direction of wave propagation. These waves are commonly generated by devices like magnetrons (used in microwave ovens) and klystrons (used in radar systems).
Microwaves interact with matter based on their frequency:
- Lower-frequency microwaves pass through obstacles and are used in long-distance communication.
- Higher-frequency microwaves are absorbed by materials, making them useful for heating applications.
Uses of Microwaves
| Application | Description |
|---|---|
| Communication | Used in satellite, Wi-Fi, Bluetooth, and mobile networks due to their ability to penetrate clouds and atmosphere. |
| Radar Systems | Used in weather forecasting, air traffic control, military surveillance, and speed detection (police radar). |
| Cooking | Microwaves excite water molecules in food, generating heat and cooking food efficiently. |
| Medical Use | Used in diathermy for deep tissue heating and microwave imaging techniques. |
| Astronomy | Cosmic Microwave Background (CMB) radiation helps scientists study the early universe. |
| Navigation | GPS systems rely on microwave signals from satellites to provide accurate location tracking. |
Microwaves in Everyday Life
- Microwave Ovens: The 2.45 GHz frequency is commonly used in kitchen microwave ovens because it efficiently interacts with water, fats, and sugars, heating food quickly.
- Wireless Communication: Microwaves are used in Wi-Fi (2.4 GHz and 5 GHz bands), Bluetooth, and mobile phone networks (4G, 5G) for data transmission.
- Radar Technology: Weather radars use microwaves to track storms, while military radars detect enemy aircraft and missiles.
Microwave Safety
- Microwaves do not ionize atoms, so they are generally safer than X-rays and gamma rays.
- However, prolonged exposure to high-power microwaves can cause tissue heating, leading to burns or other health risks.
- Microwave ovens are designed with metal shielding to prevent leakage and ensure safety.
Thus, microwaves play a crucial role in modern technology, from cooking to communication and scientific research.
wave length chart
Wavelength Chart of the Electromagnetic Spectrum
The electromagnetic spectrum consists of different types of waves classified by their wavelengths (λ\lambda) and frequencies (f). The table below shows the wavelength ranges of various electromagnetic waves.
| Type of Wave | Wavelength Range | Frequency Range |
|---|---|---|
| Radio Waves | > 1 m to 100 km+ | < 300 MHz |
| Microwaves | 1 mm – 1 m | 300 MHz – 300 GHz |
| Infrared (IR) | 700 nm – 1 mm | 300 GHz – 430 THz |
| Visible Light | 400 nm – 700 nm | 430 THz – 750 THz |
| Ultraviolet (UV) | 10 nm – 400 nm | 750 THz – 30 PHz |
| X-Rays | 0.01 nm – 10 nm | 30 PHz – 30 EHz |
| Gamma Rays | < 0.01 nm | > 30 EHz |
Key Points About Wavelengths:
- Radio waves have the longest wavelengths (can be thousands of kilometers long).
- Gamma rays have the shortest wavelengths (less than 0.01 nanometers).
- Visible light is a small portion of the spectrum that humans can see.
- Higher frequencies correspond to shorter wavelengths and higher energy.
communications satellites transmit uv waves. true or false
False.
Communication satellites do not transmit ultraviolet (UV) waves. Instead, they use radio waves and microwaves, which can travel long distances and penetrate the Earth’s atmosphere without significant absorption.
- Radio waves (e.g., AM/FM signals, TV broadcasts, GPS)
- Microwaves (e.g., satellite communication, mobile networks, radar)
UV waves are not used in communication because they have high energy, are easily absorbed by the Earth’s atmosphere, and do not efficiently carry signals over long distances.
do digital x rays use radiation
Do Digital X-Rays Use Radiation?
Yes, digital X-rays use radiation, just like traditional film-based X-rays. They rely on X-ray radiation, which is a type of high-energy electromagnetic wave with short wavelengths (0.01–10 nm). However, digital X-rays are designed to use lower doses of radiation while still producing high-quality diagnostic images.
How Digital X-Rays Work
- X-ray Beam Generation
- A controlled X-ray beam is emitted from an X-ray tube.
- This beam consists of ionizing radiation that can pass through soft tissues but is absorbed by denser structures like bones.
- Passing Through the Body
- Different body tissues absorb X-rays at different rates.
- Bones absorb more X-rays, appearing white on the image.
- Soft tissues absorb fewer X-rays, appearing in shades of gray.
- Air-filled areas (like lungs) allow most X-rays to pass through, appearing dark.
- Detection by Digital Sensors
- Instead of using traditional film, digital detectors (flat-panel or phosphor plates) capture the X-rays that pass through the body.
- These detectors convert X-ray energy into electronic signals, which are then processed into digital images.
- Image Processing and Storage
- The captured image is displayed on a computer screen in real time.
- Radiologists can adjust contrast, zoom in, and enhance images for better diagnosis.
- The digital format allows for easy storage, sharing, and retrieval in PACS (Picture Archiving and Communication System).
Radiation Exposure in Digital X-Rays
Compared to traditional X-ray methods, digital X-rays reduce radiation exposure by 50–80% due to:
- Higher sensitivity of digital sensors, requiring less radiation to produce clear images.
- Better image processing, reducing the need for repeated scans.
- Automatic exposure control (AEC), adjusting radiation levels based on body part density.
Despite these advancements, X-rays are still ionizing radiation, meaning excessive exposure over time could potentially cause cellular damage. However, the doses used in medical imaging are very low and considered safe under controlled conditions.
Advantages of Digital X-Rays
| Feature | Digital X-Rays |
|---|---|
| Radiation Dose | Lower than traditional X-rays (50–80% reduction). |
| Image Quality | High-resolution with digital enhancement. |
| Processing Time | Instant image display (no film development needed). |
| Storage & Sharing | Easy digital storage and remote access via PACS. |
| Environmental Impact | No chemicals or film waste. |
| Repeatability | Fewer retakes needed due to image adjustments. |
Are Digital X-Rays Safer?
Yes, digital X-rays are safer than traditional X-rays due to reduced radiation exposure, higher efficiency, and faster imaging. However, as with any form of ionizing radiation, unnecessary exposure should be minimized. Protective measures, such as lead aprons and thyroid shields, are often used to protect sensitive areas from radiation.
Thus, while digital X-rays still use radiation, they are more efficient, safer, and environmentally friendly compared to conventional film-based X-ray systems.
Frequently Asked Questions :
1. What are long-wavelength radio waves used for?
Long-wavelength radio waves are used for AM/FM radio broadcasting, aviation communication, and military transmissions.
2. How do long-wavelength radio waves help in emergency situations?
1. What are long-wavelength radio waves used for?
Long-wavelength radio waves are used for AM/FM radio broadcasting, aviation communication, and military transmissions.
3. Can long-wavelength radio waves penetrate water?
Yes, they can penetrate water, making them ideal for submarine communication.
4. Why are long-wavelength radio waves used in astronomy?
Astronomers use them in radio telescopes to study celestial objects that emit low-frequency radio signals.
5. How do long-wavelength radio waves support GPS systems?
They are used in time signal transmission to help synchronize clocks and improve GPS accuracy.
Also Read :
Electromagnets: Powering the Future with Magnetic Force
Electromagnetic Fields: 2 Types, Working Principles, and Applications